Determining the Enthalpy of a Chemical Reaction Pre Lab Answers
In order to calculate the change in enthalpy per mole divide the total heat by the number of moles of each reactant. The enthalpy changes are.

Determining The Enthalpy Of A Chemical Reaction
Two chemicals were poured into a Styrofoam cup and a temperature probe.
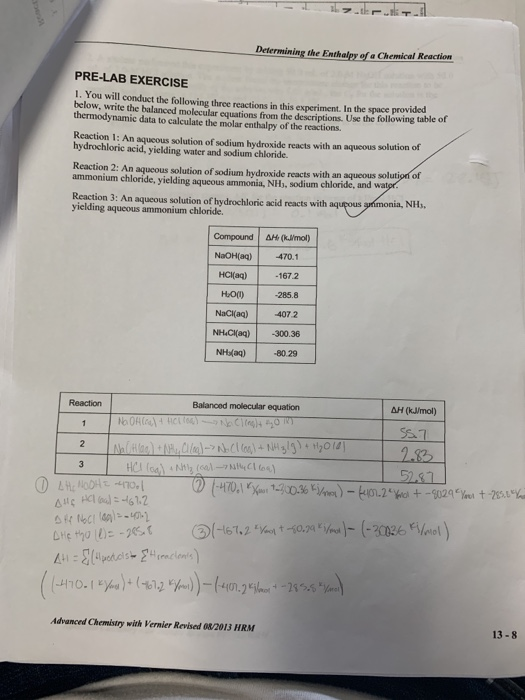
. Use the following table of thermodynamic data to calculate the molar enthalpy of the reactions. Enthalpy change of a chemical reaction lab answers. The variable q represents the heat energy that is gainedlost Cp is the specific heat of water m is the mass of water and T is the temperature change of the reaction.
In the space below write the balanced molecular equations from the thermodynamic data to calculate the molar enthalpy of the reactions. We only have the error which can be due to the experiment record and uncertain. Download the Android app.
In the space provided below write the balanced molecular equations from the descriptions. Use Hesss law to determine the enthalpy change ΔH of a third reaction reaction bw aq ammonia and aq hydrochloric. The mass m can be found by using the density m d x Vtot total volume.
Calculations Enthalpy equation o qmC T Hesss Law o H 3 H 1- H 2 kJmol q1 Sample Calculations Temperature Change a o Part 1 363 C 218 C 145 C o Part 2 224 C 215 C 09 C 224 C 211 C 13 C o Part 3 337 C 208 C 129 C q surroundings b. Revised 111517 Page 1 of 2 NAME_____STATION _____DATE_____ CHM 111 DETERMINE THE ENTHALPY OF CHEMICAL REACTION - HESSS LAW PRE-LAB 1. You will conduct the following three reactions in this experiment.
The term q represents the heat energy that is gained or lost. Use the table of thermodynamic data in your text or. To determine the enthalpy one can use the following.
Moles MassMolar Mass Hesss Law. You will conduct the following three reactions in this experiment. The moles of each reactant is determined by dividing the mass of the reactant by the molar mass of the reactant.
Chemistry questions and answers. Learn vocabulary terms and more with flashcards games and other study tools. In order to calculate the change in enthalpy per mole divide the total heat by the number of moles of each reactant.
If we conduct a reaction between two substances in aqueous solution then the enthalpy of the reaction can be indirectly calculated with the following equation. The enthalpy change of the reaction 3 which is -3723Kjmol was different to actual enthalpy change -522 Kjmol. What is the enthalpy change for the reaction.
You will conduct the following three reactions in this experiment. Study on the go. Enthalpy Lab Manual on Angel - - 150655-XUG3 Appendix.
A more universal value is that of enthalpy which is reported in kJmole of a particular reactant which in this case will be the limiting reagent. Enthalpy is the heat content of a system. Determining the enthalpy of a chemical reaction pre lab answers.
The amount of heat absorbed or released under this condition is the enthalpy change H for the reaction where H H products - H reactants 9-1 Enthalpy H can be thought of as the heat content of a substance. To determine the enthalpy one can use the following. Thermodynamics enthalpy of reaction and hesss law post lab answers.
The heat evolved for a chemical reaction can be determined by running the reaction in a calorimeter and measuring the temperature change. By measuring the temperature change the enthalpy of the reaction can be calculated through the equation q Cp m T. The heat effect for a chemical reaction run at constant pressure such as those run on the bench top in open vessels is the enthalpy change in kJmol times the amount mol of reaction q rxn nH.
- The experimental molar enthalpy for Reaction 3 is -4047 kJmol - ΔH -4348 kJmol 3014 kJmol -4047 kJmol 4. The purpose of this lab is to measure the temperature change of several reactions. The mass m can be found by using the density m d x Vtot total volume.
Abstract In this experiment enthalpy of a chemical reaction will be determined. Use Hesss law and the accepted values of ΔH in the Pre-Lab Exercise to calculate the ΔH for Reaction 3. Enthalpy of a chemical reaction lab answers.
The enthalpy change of a reaction is roughly equivalent to the amount of energy lost or gained during the reaction. The moles of each reactant is determined by dividing the mass of the reactant by the molar mass of the reactant. All chemical reactions involve an exchange of - follow a reaction by measuring the - H.
Chemistry questions and answers. Determining the enthalpy of a chemical reaction lab answers. Use your answers from 2 above and Hesss law to determine the experimental molar enthalpy for Reaction 3.
The experiment supports Hesss law. Cp is the specific heat of water m is the mass of water and T is the temperature change of the reaction mixture. Use the specific heat of water 418 JgC for all solutions.
Q n 10 where q is the heat change of the reaction and n is the number of moles of the limiting reagent. The enthalpy values can then be compared to the same reaction performed with different amounts of a substance or to accepted enthalpy values. LAB 9 Determining the Enthalpy Of a Chemical Reaction Pawel Klos 4815 1.
This heat is stored as potential energy in the form of bond and other energies. Start studying Chemistry Enthalpy Lab. Moles MassMolar Mass Hesss Law.
D 10 where q is the heat change of the reaction and n is the number of moles of the limiting reagent. Input all of these values to the equation ΔH ΔQ p ΔV to obtain the change in enthalpy. Calculate the enthalpy change H for each reaction in terms of kJmol of each reactant.
Determining the Enthalpy of a Chemical Reaction PRE-LAB EXERCISE 1. Kjmol 49 Kjmol -522 Kjmol Percentage of error -5223723x1005222867 5. A reaction is favored if the enthalpy of the.
That are open to the atmosphere. In the space provided below write the balanced net ionic reaction equations from the descriptions. Enthalpy of reaction and hesss law lab answers.
Determining the Enthalpy of a Chemical Reaction PRE-LAB EXERCISE 1. Use your answers from 2 above and Hesss law to determine the experimental molar enthalpy for Reaction 3. Q 418 103 18 508 q 418 103 1 4736 q 418 103 34 146 We can then calculate the enthalpy change for each reaction.
Use 103 gmL for the density of all solutions.

Solved Pre Lab Determining The Enthalpy Of A Chemical Chegg Com

Solved Chm 111 Determine The Enthalpy Of Chemical Reaction Chegg Com

Solved Determining The Enthalpy Of A Chemical Reaction Chegg Com

Comments
Post a Comment